Abstract
The dysmetabolic cycle in the gastrointestinal physiology of type 2 diabetes mellitus, triggered by the chronic consumption of high-glycemic index and high-fat diets, includes amplified digestion and a consequent increase of nutrient absorption of breakdown products of carbohydrates, lipids and proteins. The resultant chronic exposure of tissues to high plasma levels of glucose, fatty acids and amino acids causes tissue resistance to the actions of insulin and, at a later stage, β-cell dysfunction and reduction of insulin release. The hyperinsulinemia increases hunger, feeding back on the overconsumption.
The amplified digestion presupposes both increased vagally and hormonal (-cholecystokinin and secretin- mediated) stimulation of biliopancreatic secretion.
Considering the amplified digestion as the main target for the correction of the dysmetabolic cycle of type 2 diabetes, we propose that a dedicated antidiabetic surgery should include both:
- truncal vagotomy for the reversal of the increased vagal parasymathetic activity, and
- bypassing the passage of food through the duodenum and proximal jejunum in order to avoid nutrient-overstimulation of cholecystokinin- and secretin- secreting cells, which are most abundant in this area of the intestine.
The ‘diabetogenic’ potential of the modern dietary patterns!
The association between the modern dietary pattern of Western diet with the increased risk of chronic diseases, such as obesity and type 2 diabetes, is widely accepted (1). The high intake of processed and refined foods, added sugars, and saturated and trans fats and low intakes of fruits, vegetables, whole grains, and nuts has been linked to an increased risk of type 2 diabetes (2, 3). Τhe high glycemic index or glycemic load, as well as the high-fat diets, predispose to higher postprandial blood glucose and insulin concentrations, insulin resistance and, at a later stage, type 2 diabetes (4–8). The strong relation between diet composition and the onset of diabetes is also evident from the use of high-fat diets, which induce insulin resistance, type 2 diabetes and obesity in animal models (9, 10).
The pathophysiological mechanisms by which the macronutrients lead to the development of type 2 diabetes are in our research interests and we explain them below.
Amplified digestion: the trigger point of the dysmetabolic cycle of type 2 diabetes
The absorption of the main types of dietary macronutrients -carbohydrates, fat and proteins- requires their breakdown, through digestion into absorbable forms, the oligo-, mono-saccharides, free fatty acids and amino acids, respectively (11). The enzymes secreted from exocrine pancreas, in particular amylase, lipase, trypsin, and chymotrypsin, play a major role in the digestion process. Several enzymes in the brush border of the small bowel are also involved in macronutrient digestion. Moreover, bile acids, the major lipid components of bile, are critical for the digestion and absorption of lipids.
The pancreatobiliary digestive secretory mechanism is adapted to the amount and type of dietary components (12). It is clear that the secretion of the enzymes involved in digestion of protein, carbohydrate and lipid adapts to any change in substrate intake. For instance, when the amount of starch intake increases, the specific activity of pancreatic amylase is stimulated. Therefore, the high glycemic index and load diets increase pancreatic and intestinal brush border enzyme activities, leading to amplified digestion (13). It should be noted, that the strongest stimulants of pancreatic enzyme secretion are the lipids and that chronic administration of a diet with a high fat content is permanently associated with a high enzyme output compared with diets rich in carbohydrates or proteins (12, 14). Τhe high-fat diet also increases bile acid secretion.
Thus, the amplified digestion due to chronic consumption of the high fat and high glycemic index/ load diets is a key step in the vicious dysmetabolic cycle that leads to type 2 diabetes (13).
Research data in favor of enhanced digestion in type 2 diabetes mellitus
I.The elevated pancreatic enzyme activities in type 2 diabetes. Several studies indicate that type 2 diabetes stimulates pancreatic enzyme secretion . Indeed, in the large LEADER study, 23 % of 9340 patients with type 2 diabetes had elevated fasting amylase and lipase activity levels (15), while similar results were reported by Malloy J. et al (16). Also, Srihardyastutie A et al. showed a significant positive correlation between increased serum lipase and HbA1c levels, insulin resistance, and duration of diabetes (17), while Rahman et al. demonstrated that amylase levels correlated positively with blood glucose level, duration of diabetes, and Body Mass Index (BMI) (18).
The importance of pancreatic enzyme activity is also evident from the use of amylase and lipase enzyme inhibitors as potential therapeutic tools in the management of type 2 diabetes mellitus and/or obesity. This pharmacologic manipulation leads to a decrease in blood glucose levels, improvement of oral glucose tolerance, slowing down of the rate of type 2 diabetes progression and body weight loss (19–23).
II. The elevated hydrolytic brush border enzyme activities in type 2 diabetes. That the activities of disaccharidases, including sucrase and isomaltase, are abnormally high in the small intestine of both experimental animals and patients with type 2 diabetes, has been demostrated by a series of studies for over 50 years (24, 25). The alpha-glucosidase inhibitors, as therapeutic agents in managing type 2 diabetes, reveal the importance of the small bowel brush border enzymatic activity in the onset of the disease (26).
IΙΙ. The bile acid sequestrans in the treatment of type 2 diabetes. In addition to their significant lipid-lowering properties, data from several studies suggest that, bile acid sequestrans might also improve glycemic control in patients with type 2 diabetes (27, 28) The important role of bile acids in the digestive process in type 2 diabetes, is demonstrated by the use of bile acid sequestrans, not only as significant lipid-lowering agents, but also for improving glycemic control in patients with type 2 diabetes.
The importance of increased nutrient absorption in type 2 diabetes.
The amplified digestion of carbohydrates, fats and proteins leads to amplified absorption of their respective breakdown products in type 2 diabetes. Indeed, increased glucose absorption, as well as expression and activity of specific glucose transporters in enterocytes are present in type 2 diabet (29, 30). Furthermore, clinical data have shown that intestinal permeability and lipid absorption may also be increased in type 2 diabetes. Although to date this phenomenon has been attributed to the dysbiosis of gut microbiota, we suggest that it is the main result of the amplified lipid digestion observed in type 2 diabetes (31).
The establishment of the ‘insulin resistance stage’ of type 2 diabetes.
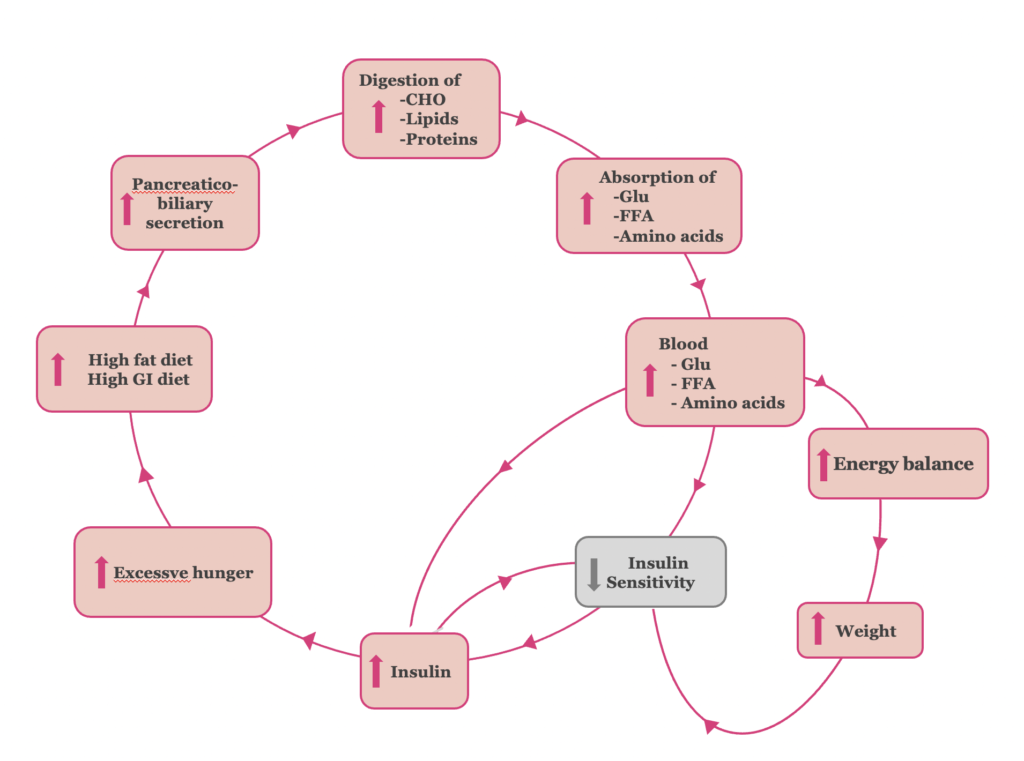
The chronic exposure of tissues to amplified absorption of glucose, fatty acids and amino acids, leads initially to tissue resistance to the actions of insulin and, at a later stage, β-cell dysfunction, reduction of insulin release and type 2 diabetes mellitus. We suggest that the main mechanisms contributing to the insulin resistance are the following (Figure 1):
Ι. The elevated blood glucose levels lead to a compensatory increase of insulin secretion by pancreatic β-cells, to maintain normal plasma glucose levels. Τhe hyperinsulinemia contributes to the induction of insulin resistance, and vice versa (32). The adaptation of β-cells to the increased insulin demands constitutes the initial ‘adaptation stage’ of type 2 diabetes.
II. Τhe excess of calories consumed by the high fat and high glycemic index diets and the positive energy balance resulting from the amplified absorption of glucose, fatty acids and amino acids, respectively, leads to body weight gain. Thus, obesity induced-insulin resistance is established (33, 34).
III. The hyperinsulinemia, in its turn, increases hunger, feeding back the overconsumption. (35, 36). Thus, the vicious cycle of ‘insulin resistance stage’ of type 2 diabetes’ continues.
Figure 1. The pathophysiology of the “insulin resistance” stage of T2DM.
The establishment of the ‘β-cell dysfunction stage’ of type 2 diabetes.
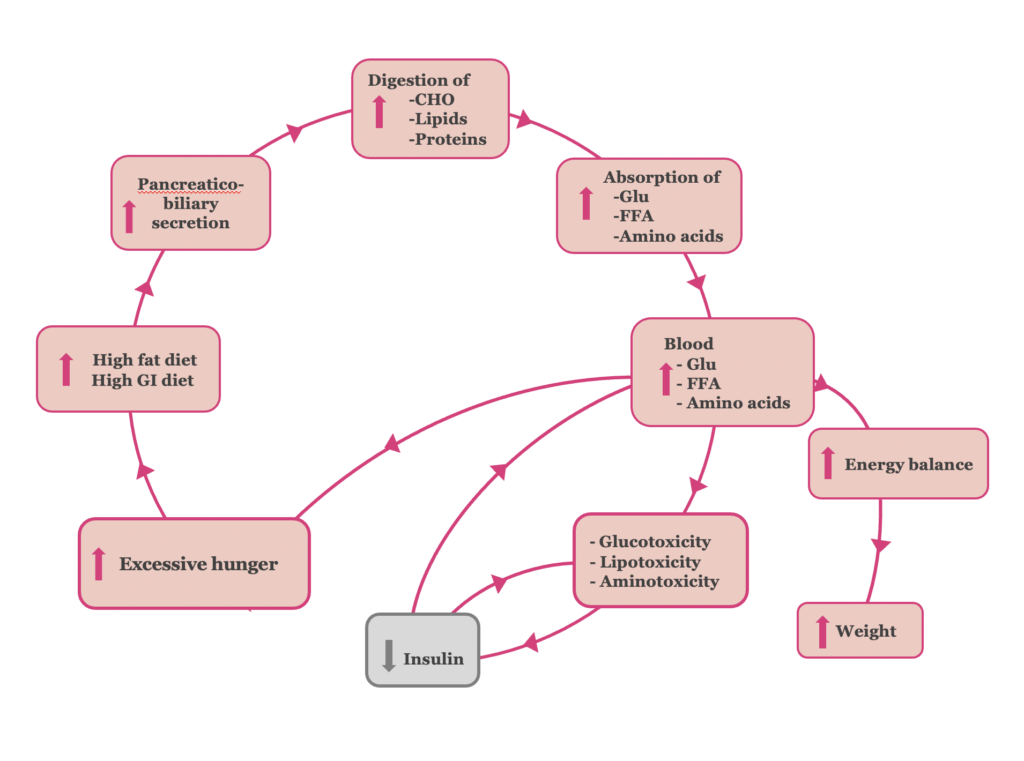
At a later stage though, pancreatic β-cell activity can no longer adequately meet the insulin demand created (Figure 2). The β-cell “hyper-stimulation” and subsequent “exhaustion” under gluco- lipo- and amino acid- toxic conditions, lead to pancreatic β-cell dysfunction and impaired insulin secretion. At the stage of β-cell dysfunction, hyperglycemia develops, and type 2 diabetes is established (37, 38).
The vicious cycle of type 2 diabetes is completed, as excessive hunger, a symptom of hyperglycemia, leads to hyperphagia.
Figure 2. The pathophysiology of the ‘β-cell dysfunction’ stage of T2DM.
Digestion: A neurohormonal gastrointestinal pathway.
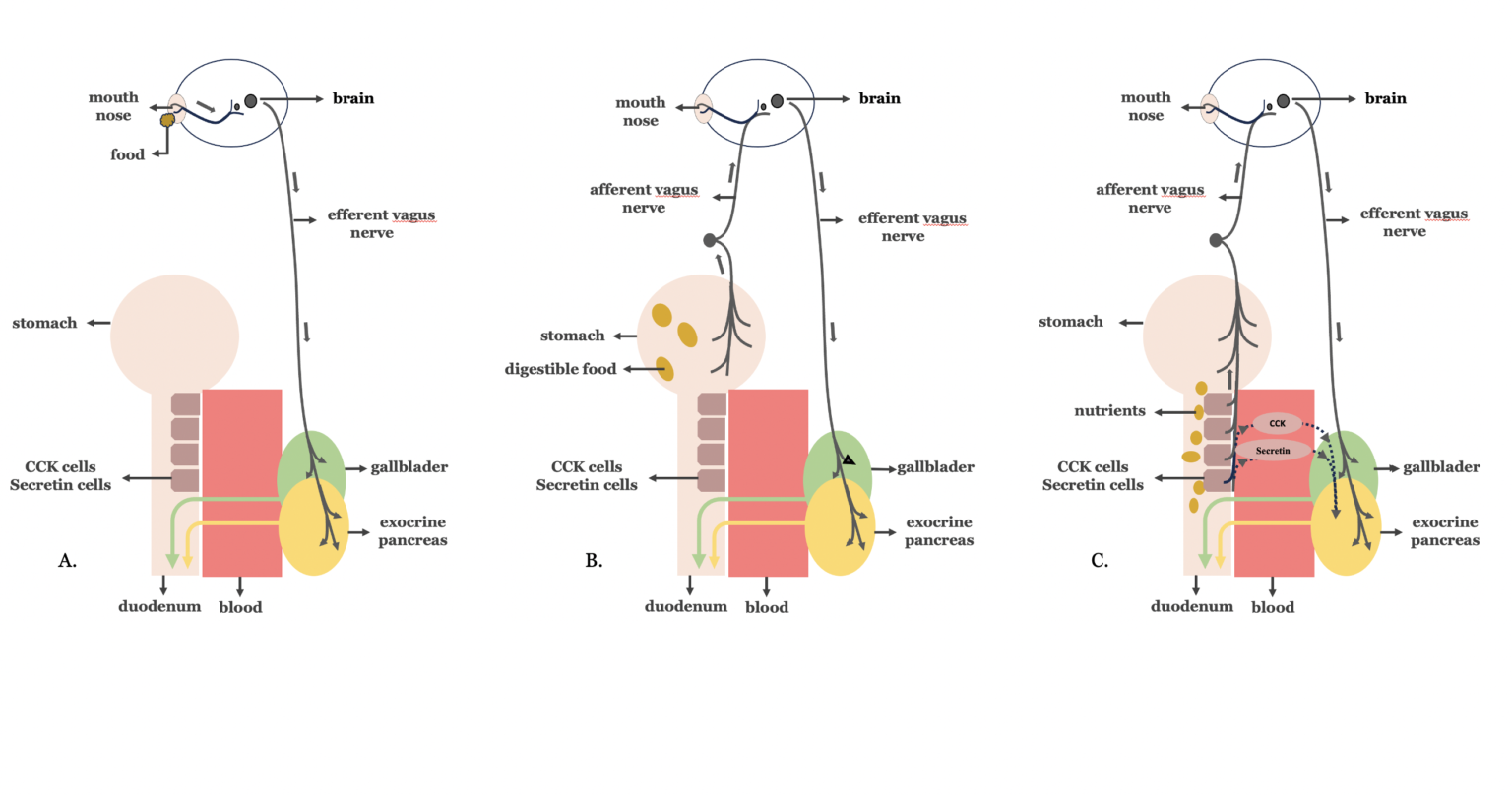
The pancreatobiliary secretion is necessary for the digestive process. It is divided into three phases, the cephalic, gastric, and intestinal phase, depending on the origin of the signals to the pancreas and the gallbladder (39). The vagus nerve, the principal component of the parasympathetic nervous system, plays a major role in the regulation of all three phases of the pancreatobiliary secretion. During the cephalic phase (Figure 3A), signals from taste receptors are transmitted through the vagus nerve to the pancreas and the gallbladder. The gastric phase (Figure 3B), is mediated mainly by vago-vagal cholinergic reflexes that originate in the stretch receptors of the stomach and terminate in the pancreas and the gallbladder. The intestinal phase is the major phase contributjng up to 70 % of the postprandial pancreatobiliary secretion (Figure 3C). Τhis phase is stimulated by the gastric chyme as it enters into the duodenum. In the duodenum, fats and proteins stimulate cholecystokinin (CCK) release from CCK-secreting cells, while gastric acid and ingested fatty acids stimulate secretin release by secretin-secreting cells, respectively. These cells are most abundant in the duodenum, while they progressively decrease in number distally, in the jejunum.
Cholecystokinin and secretin stimulate exocrine pancreatic secretion through a direct hormonal action on exocrine pancreatic cell receptors and also exhibit a paracrine mode of action via enteropancreatic vago-vagal enteropancreatic reflexes. In addition, cholecystokinin stimulates the gallbladder to contract and release bile into the duodenum by activation of long reflexes through the vagus nerve (40, 41).
Figure 3. The three phases of biliopancreatic secretion. A. Cephalic phase B. Gastric phase C. Intestinal phase
Association of enhanced digestion with increased neurohormonal gastrointestinal activity in type 2 diabetes.
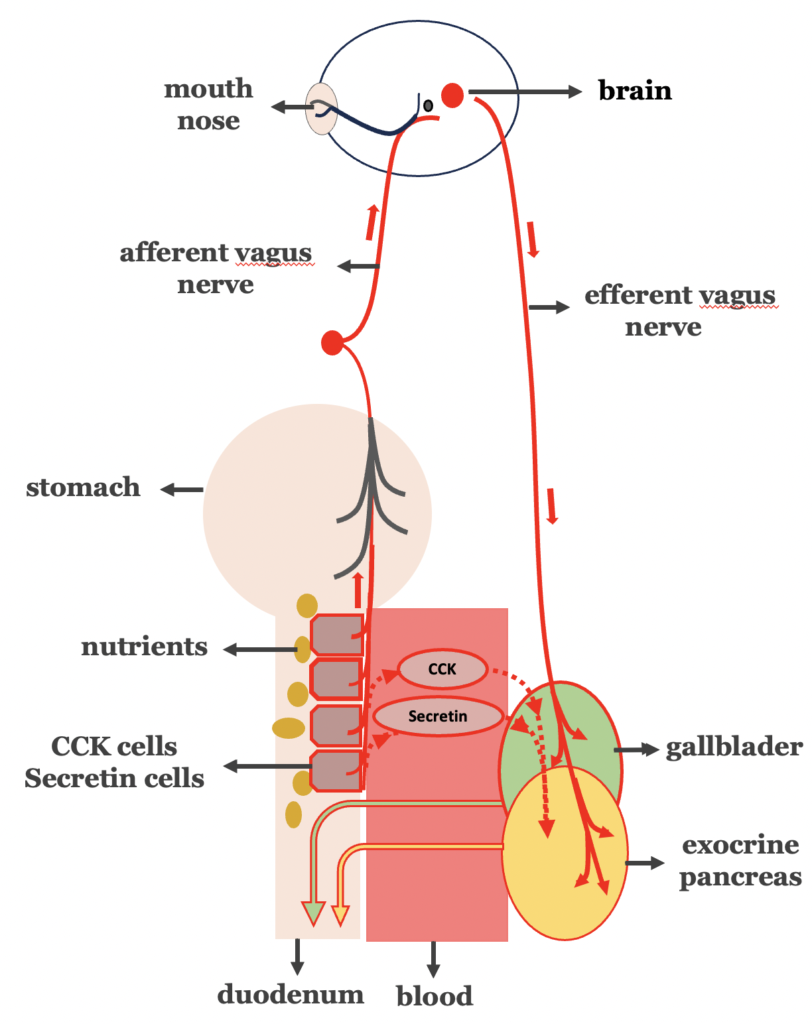
If the key step of the vicious cycle in type 2 diabetes is indeed the amplified digestion, while the digestive process is mediated both by hormonal and vagal nerve signaling pathways, the following should apply (Figure 4):
- Type 2 diabetes, should be accompanied by increased vagus nerve
- Truncal vagotomy should lead to improvement in type 2 diabetes.
- Type 2 diabetes should be linked with over-stimulation of cholecystokinin- and secretin- secreting cells, increasing hormonal mediated biliopancreatic secretion.
- Bypassing the duodenal transit of food, avoiding the stimulation of cholecystokinin- and secretin- secreting cells, should logically lead to type 2 diabetes remission.
Figure 4. Over-stimulation of the intestinal phase of biliopancreatic secretion in type 2 diabetes.
After a thorough review of the literature, we present the following impressive findings:
A. Increased vagal activity in ‘insulin resistance stage’ of type 2 diabetes.
The link between obesity, hyperinsulinemia- as a stage of insulin resistance stage of type 2 diabetes- and hyperactivity of the parasympathetic nervous system has been documented since the 1980s. Rohner-Jearenaud F, a leading researcher in this field, demostrated that the increased insulin secretion in an obese animal model, is mediated mainly by an increased vagal tone acting on the β-cells (42–44). Later, research data attributed the development and maintenance of hyperinsulinemia to an autonomic system activity imbalance, which includes increased parasympathetic and decreased sympathetic gastrointestinal nervous sustem activity (45, 46).
B. Truncal vagotomy for the improvement of insulin sensitivity in type 2 diabetes
These above findings agree with some research studies suggesting that truncal vagotomy, performed as a ‘stand-alone’ procedure for the treatment of morbid obesity, normalizes insulinemia and restores sensitivity to insulin (42, 47–49). Additionaly, truncal vagotomy has consistently been associated with reduced hunger, decreased food intake, and body weight loss in subjects with severe obesity (50). Since 1978, truncal vagotomy has been described as a potential treatment for obesity, but, so far, it has not been systematically used as such (51).
C and D. Proximal intestinal by-pass procedures most effective for the remission of type 2 diabetes.
Τhe direct contribution of the proximal intestine (duodenum and proximal jejunum) to the development of type 2 diabetes, was initially suggested by Rubino et al., based on experiments conducted in diabetic rats (52, 53). The experiments revealed that the exclusion of duodenal nutrient passage in diabetic rats significantly improved glucose tolerance, while the restoration of duodenal passage in the same rats reestablished impaired glucose tolerance!
Several human research studies have shown that bariatric surgical procedures that involve proximal intestinal by-pass are more effective on diabetes remission, compared to those without. Among 23.106 patients with metabolic syndrome, remission of type 2 diabetes was far greater in gastric by-pass and biliopancreatic diversion (62% and 74%, respectively), than in patients with sleeve gastrectomy and gastric band (52% and 28%, respectively), after one year of follow-up (54).
Similar results were also reported by other researchers in both retrospective (55) and prospective studies (56) after 2 and 3 years of follow-up, respectively. In studies with 5 and 8 years of follow-up, gastric bypass continued to be superior to sleeve gastrectomy not only in the rates of diabetes remission but also in the rates of its recurrence (57–59).
Conclusion
According to our theory (13) the key step of the dysmetabolic cycle in the gastrointestinal physiology of type 2 diabetes that results from chronic overconsumption of high-fat and high glycemic index/ load diets, is the amplified digestion of nutrients following increased intestinal absorption. The amplified digestion requires increased biliopancreatic secretion, which presupposes increased vagally- and hormonally (cholecystokinin and secretin)- mediated biliopancreatic secretion.
Considering the amplified digestion as the main target for the correction of the dysmetabolic cycle of type 2 diabetes, we propose that dedicated antidiabetic surgery should include: a. truncal vagotomy for the reversal of the increased parasympathetic activity, and b. proximal (duodenum and proximal jejunum) intestinal bypass to avoid nutrient-overstimulation of cholecystokinin and secretin cells. Our theory, entitled: ‘Metabolic effects of truncal vagotomy when combined with bariatric-metabolic surgery’, was published in one of the top specialty journals “Metabolism” in 2022 (13).
The clinical trial VagusSx combines these two procedures in one with the goal of achieving a long complete remission of type 2 diabetes. We hope that in the future, reducing pancreatobiliary digestion as a means to improve the treatment of type 2 diabetes will be approached by gentler, less invasive methods.