Abstract
We propose a novel surgical approach for the treatment of type 2 diabetes mellitus: the combination of truncal vagotomy with gastric bypass. This dual intervention is intended to produce a more profound and sustained inhibition of biliopancreatic secretion, reduce nutrient absorption, and amplify the secretion of beneficial gut hormones. By directly modulating the neurohormonal drivers of metabolic dysregulation, this approach may lead to superior glycemic control and higher rates of durable diabetes remission compared to gastric bypass alone.
The rationale for this proposal is grounded in a new theoretical framework centered on the concept of “amplified digestion”—a maladaptive physiological state triggered by chronic intake of high-glycemic and high-fat diets. This condition leads to excessive nutrient breakdown and absorption, promoting sustained hyperglycemia, hyperinsulinemia, insulin resistance, and progressive β-cell dysfunction. Amplified digestion is maintained by continuous activation of biliopancreatic secretions, which are regulated by both hormonal and neural signals.
Current bariatric-metabolic surgeries, such as gastric bypass, improve metabolic parameters in part by bypassing portions of the gastrointestinal tract. Importantly, gastric bypass inhibits the gastric and intestinal phases of biliopancreatic secretion—mainly by disrupting hormonal signaling, such as the release of secretin and cholecystokinin, which are normally stimulated by nutrient contact in the proximal small intestine—thereby reducing nutrient absorption and altering gut hormone secretion.
However, vagal stimulation remains an active driver of biliopancreatic secretion through the cephalic phase and residual neural input during the gastric and intestinal phases. Truncal vagotomy, by severing vagal input to the stomach, pancreas, and proximal intestine, suppresses these phases and more directly inhibits the secretory processes that underlie amplified digestion.
By combining truncal vagotomy with gastric bypass, we aim to intensify the metabolic benefits of surgery through a more complete suppression of digestive stimulation and nutrient absorption. This approach may enhance insulin sensitivity, improve β-cell function, and support appetite regulation through increased hormonal signaling from the distal gut.
While truncal vagotomy introduces additional surgical complexity and carries potential risks—including altered gastric motility and digestive side effects—its targeted mechanism offers a compelling opportunity for patients with early-stage type 2 diabetes and preserved β-cell function. We advocate for rigorous clinical studies to evaluate the safety, efficacy, and long-term outcomes of this combined approach in selected patient populations.
Introduction
In our previous article, we described the vicious dysmetabolic cycle in the gastrointestinal pathophysiology of type 2 diabetes mellitus. This pathogenetic pathway, triggered by the chronic consumption of high-glycemic index and high-fat diets, includes amplified digestion and a consequent increase of nutrient absorption of breakdown products of carbohydrates, lipids and proteins. The resultant chronic exposure of tissues to high plasma levels of glucose, fatty acids and amino acids causes tissue resistance to the actions of insulin and, at a later stage, β-cell dysfunction and reduction of insulin release. Both hyperinsulinemia and hyperglycemia, corresponding to the two main pathological manifestations observed during the progression of type 2 diabetes, enhance hunger, feeding back into the cycle of overconsumption. Within the above framework, ‘amplified digestion’ represents a central feature of the vicious dysmetabolic cycle underlying type 2 diabetes; consequently, its inhibition constitutes a primary therapeutic target in the management of the disease.
This reasoning constitutes our original theory regarding the mechanism and treatment of type 2 diabetes, which, under the title:‘Metabolic effects of truncal vagotomy when combined with bariatric-metabolic surgery’, was first published in one of the top specialty journals ‘Metabolism’ in 2022 (1).
Acceptance of this theory leads to the following conclusions:
- Given that bariatric-metabolic surgical procedures are associated with varying degrees of improvement in type 2 diabetes, it is reasonable to infer that they achieve this through correspondingly variable degrees of inhibition of enhanced digestion.
- Since the digestive process is mediated by biliopancreatic secretion, bariatric-metabolic surgical procedures are consequently associated with varying degrees of inhibition of this secretion(2).
- The greater the inhibition of biliopancreatic secretion induced by a bariatric-metabolic surgical procedure, the more pronounced its effectiveness in improving type 2 diabetes.
- The design of a diabetes-specific surgical intervention should aim to achieve maximal reduction of biliopancreatic secretion.
- Since amplified digestion requires both enhanced vagal stimulation and hormonally- via cholecystokinin and secretin-mediated activation of biliopancreatic secretion, a dedicated antidiabetic surgical approach should include the following (1): a) Τruncal vagotomy for the reversal of the increased vagal parasymathetic activity, and b)Surgical bypass of the proximal intestine to prevent nutrient-induced overstimulation of cholecystokinin and secretin secreting cells, which are most abundantly located in this segment of the intestine.
Regulation of pancreaticobiliary secretion
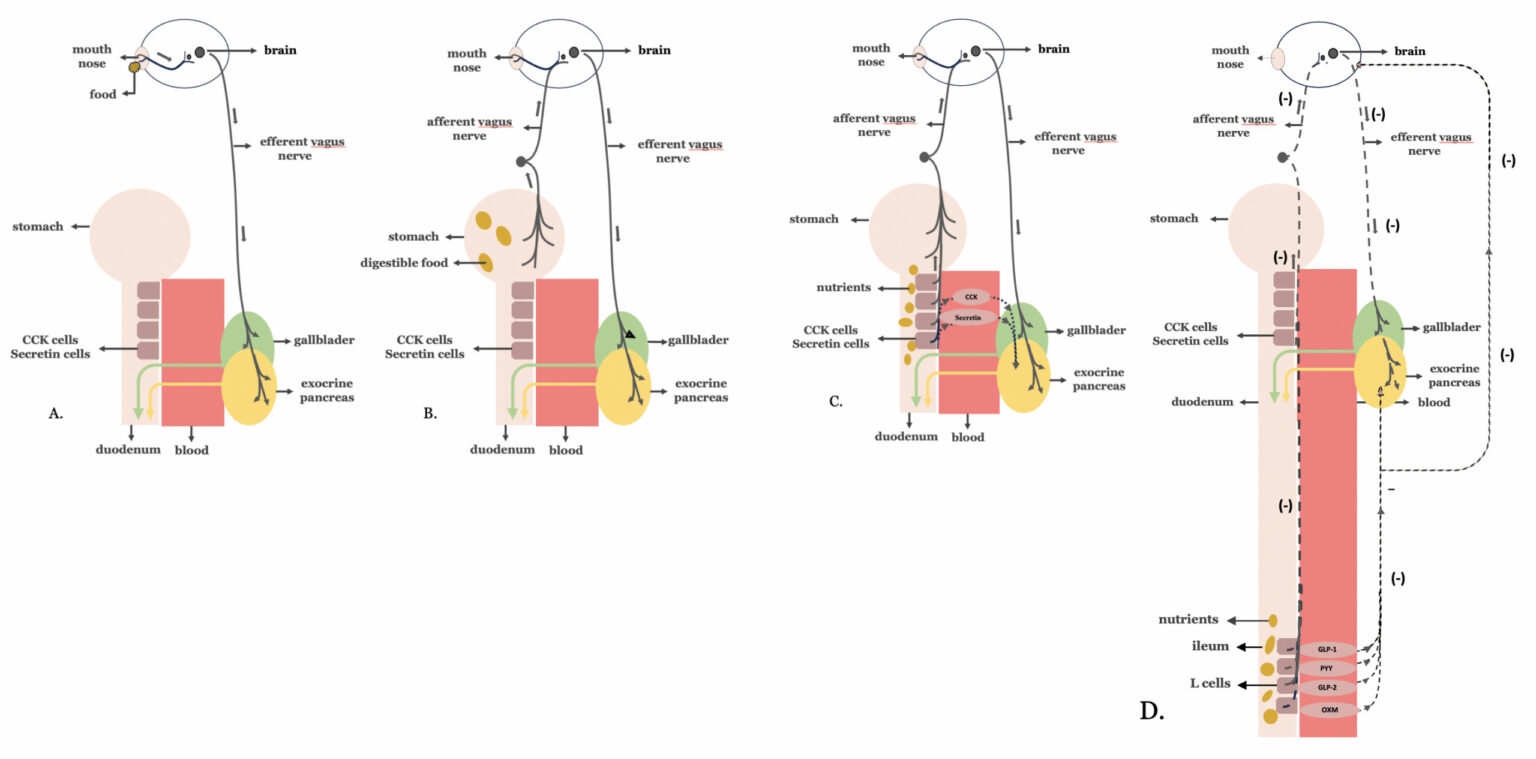
The pancreaticobiliary secretory response to a meal has been described in our previous article, entitled ‘The vicious dysmetabolic cycle of type 2 diabetes mellitus’. This refers to the three phases—cephalic, gastric, and intestinal—of a neurohormonal response triggered by the passage of food through the mouth, stomach, and proximal intestine, respectively (2).
However, when food reaches the distal intestine, a negative neurohormonal feedback mechanism is activated, suppressing exocrine pancreatic secretion—an effect known as the ‘ileal brake. The ‘ileal brake’ is a physiological response arising from nutrient-induced stimulation of L-cells located in the distal intestine (figure 1).
Figure 1. Regulation of biliopancreatic secretion: Biliopancreatic secretion, essential for digestion, occurs in three coordinated phases—cephalic, gastric, and intestinal—all regulated by neurohormonal signals in response to food intake: A.Cephalic Phase: Triggered by the sight, smell, taste, or thought of food, this phase involves vagal (parasympathetic) stimulation that primes the pancreas and biliary system for digestion before food even enters the stomach. B.Gastric Phase: Initiated when food reaches the stomach, further vagal stimulation and local hormones enhance pancreatic and biliary secretions to support ongoing digestion. C.Intestinal Phase: When food enters the duodenum, the presence of nutrients (especially fats and proteins) stimulates the release of hormones such as cholecystokinin and secretin, which further amplify pancreatic enzyme and bile secretion for digestion in the small intestine. D.Negative Feedback Mechanism – The Ileal Brake: Once nutrients reach the distal intestine (ileum), L-cells are activated to release hormones such as GLP-1, PYY, GLP-2 and oxyntomodulin. These hormones signal the suppression of further pancreatic exocrine secretion and slow gastrointestinal motility. This response—known as the ileal brake—serves as a physiological feedback loop to limit overdigestion, regulate nutrient absorption, and prevent excessive biliopancreatic activity.
Antidiabetic effects resulting from the inhibition of biliopancreatic secretion following bariatric-metabolic surgery.
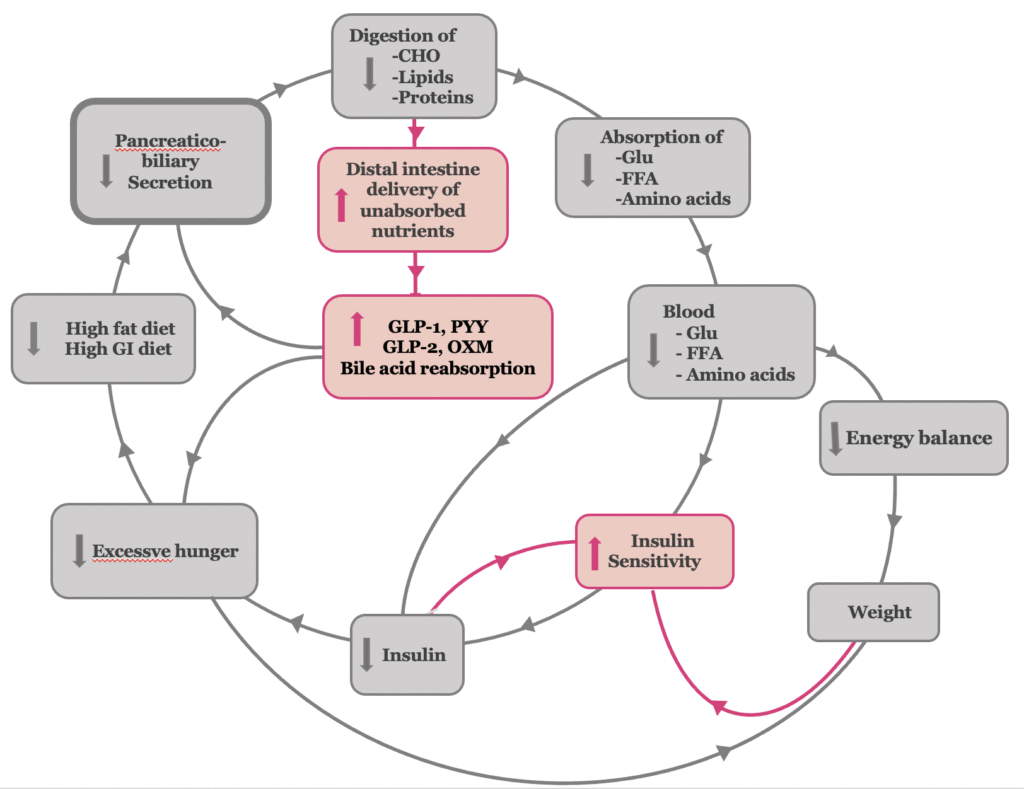
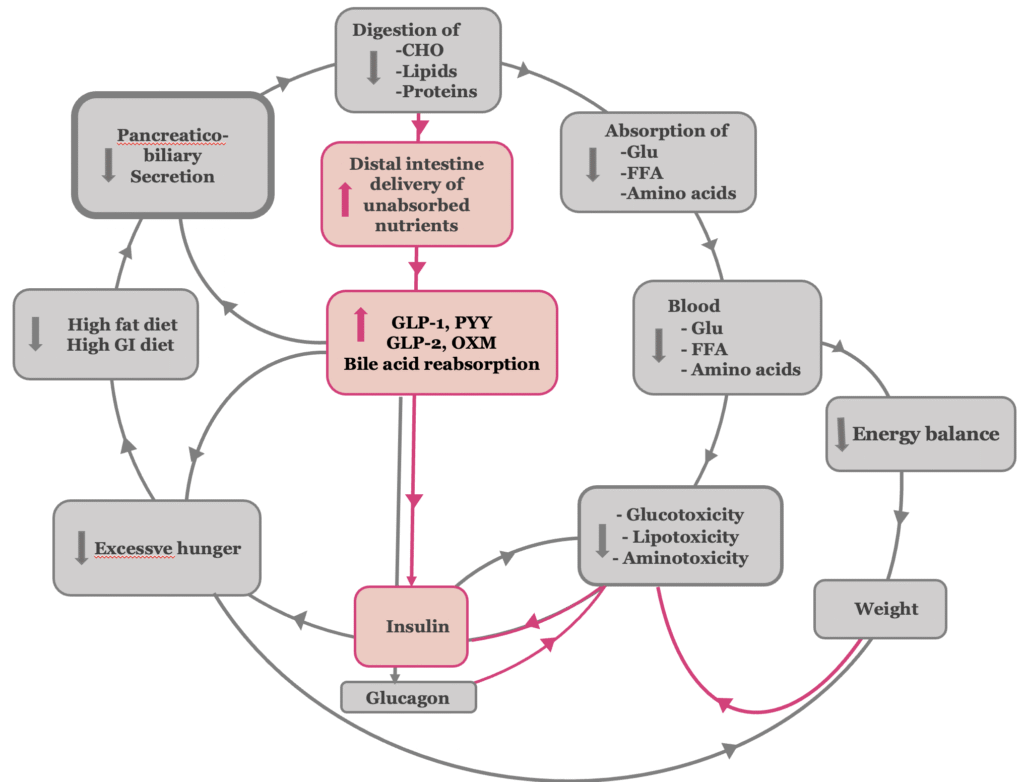
Postoperative inhibition of biliopancreatic secretion reduces the type 2 diabetes-related amplified digestion of carbohydrates, fats, and proteins in the proximal intestine, thereby decreasing the absorption of their respective breakdown products (3, 4) The initial reduction in glucose absorption, along with the subsequent decline in hyperinsulinemia, contributes to the improvement of insulin sensitivity (5) . The presence of undigested carbohydrates in the gut—acting as low-glycemic index and load nutrients—together with reduced absorption of free fatty acids and amino acids, independently leads to an improvement in insulin sensitivity(6–8). In the later stages of type 2 diabetes, the reduction of glucose-, lipid-, and amino acid–induced toxicity reverts pancreatic islet β-cell dysfunction, thereby leading to the restoration of normal insulin secretion (Figure 2,3).
The reduction of amplified digestion associated with type 2 diabetes results in the enhanced delivery of undigested food components and bile to the distal gastrointestinal tract, over-stimulating the L-cells of the distal intestine. The excessive stimulation of L-cells has the following effects:
- It increases the release of the gastrointestinal hormones Glucagon-like Peptide-1 (GLP-1), Glucagon-like Peptide (GLP-2), Peptide YY (PYY) and oxyntomodulin (9, 10). Their increased levels contribute to appetite suppression and satiety, promoting body weight loss (11). In addition, GLP-1, as an incretin, improves β-cell function by exerting trophic and anti-apoptotic effects on these cells, and enhances insulin secretion and tissue sensitivity to its actions. Furthermore, GLP-1 inhibits glucagon secretion, contributing to suppression of hepatic glucose production (12). Also, the associated elevated concentrations of PYY, oxyntomodulin and GLP-2 exert beneficial metabolic effects and further improve glucose metabolism (13–15).
- Moreover, the exposure of the ileum to undigested chyme may provide a pausible explanation for the elevated circulating bile acid levels observed after bariatric procedures, which contribute to improved glucose homeostasis (16, 17). Increased luminal exposure to bile acids enhances their reabsorption in the ileum, thereby elevating systemic bile acid levels. This supports the hypothesis that bile acid bioavailability may play a causal role in the metabolic improvements seen post-surgery. In procedures such as Roux-en-Y gastric bypass and biliopancreatic diversion, the shortened route of the enterohepatic circulation—particularly through the biliopancreatic and common limbs—accelerates the interaction between luminal bile acids and the ileum. As a result, bile acid uptake occurs earlier and more actively, contributing further to the observed metabolic benefits of incraesed bile acid levels.
- Activation of the ‘ileal brake,’ which serves as the principal negative feedback mechanism regulating exocrine pancreatic secretion, thereby completing the virtuous cycle of metabolic improvement.
Figure 2. The antidiabetic mechanism of bariatric-metabolic surgery at the insulin resistance stage of type 2 diabetes
Figure 3.The antidiabetic mechanism of bariatric-metabolic surgery at the β-cell dysfunction stage of T2DM
Evidence of Decreased Pancreatic Secretion After Bariatric Surgery
The decrease of exocrine pancreatic secretion in response to feeding after gastric bypass procedures has been described in animals since 1971 (18). In recent years, it has been also studied in humans, ranging from decreased exocrine pancreatic secretion to decompensated exocrine pancreatic insufficiency (19, 20). O’Keefe S et al. recently demonstrated significantly lower food-stimulated pancreatic secretion rates of trypsin, amylase and lipase in subjects with obesity following gastric bypass than in healthy controls (21). The impaired exocrine pancreatic secretion resulted in some fat malabsorption in all patients and frank steatorrhea in some. Recently, Ozmen M et al. showed that malabsorption caused by bariatric gastrointestinal bypass procedures can be corrected with pancreatic enzyme replacement therapy in all patients (22).
In a 52-month follow-up study, 31% of 188 consecutive patients who underwent gastric bypass, were diagnosed with exocrine pancreatic insufficiency (23). In a prospective, comparative study among 105 patients, exocrine pancreatic insufficiency was present in 75% of patients after biliopancreatic diversion, 8.3% after gastric bypass, and 4.3% after sleeve gastrectomy (24). In a study including 22 patients, 9.1% of them developed exocrine pancreatic insufficiency one year after gastric bypass (25).
The Impact of Biliopancreatic Secretion Inhibition on the Effectiveness of Bariatric-Metabolic Surgery in Improving Type 2 Diabetes
Based on the physiology of exocrine pancreatic secretion, we hypothesize that each type of bariatric-metabolic surgery procedure differentially affects the phases of biliopancreatic secretion. In general, the greater the inhibition of biliopancreatic secretion, the higher the rate of type 2 diabetes remission and the lower the incidence of relapse.
In adjustable gastric banding, the stomach as well as the proximal and distal segments of the intestine remain anatomically intact, preserving normal nutrient transit through the gastrointestinal tract and thereby failing to inhibit exocrine pancreatic secretion. Thus, gastric banding demonstrates the least therapeutic efficacy among bariatric procedures for type 2 diabetes mellitus. Actually, it improves diabetes in subjects with obesity only in the context of the body weight loss and caloric restriction. Sleeve gastrectomy, while preserving normal intestinal anatomy, inhibits the gastric phase of exocrine pancreatic secretion due to its partial resection of the stomach. Thus, sleeve gastrectomy is superior to gastric banding in terms of type 2 diabetes remission and relapse. In gastric bypass, ingested food bypasses the majority of the stomach and proximal intestine, entering directly into the distal gut. Consequently, gastric bypass inhibits biliopancreatic secretion by attenuating both the gastric and intestinal phases of secretion. In gastric bypass, the food bypasses most of the stomach and the proximal intestine and enters directly into the distal gut. The greater inhibition of exocrine pancreatic secretion observed with gastric bypass, compared to sleeve gastrectomy, contributes to its superior efficacy in achieving remission of type 2 diabetes mellitus.
The rationale for truncal vagotomy combined with gastric bypass
Focusing on gastrointestinal interventions that could maximize the reduction of biliopancreatic secretion and counteract its pathological overstimulation in type 2 diabetes mellitus, we propose investigating the addition of truncal vagotomy to gastric bypass procedures through well-designed randomized clinical trials. The primary objective is to achieve deeper, more sustained improvements in glycemic control by targeting both neural and hormonal pathways involved in amplified digestion.
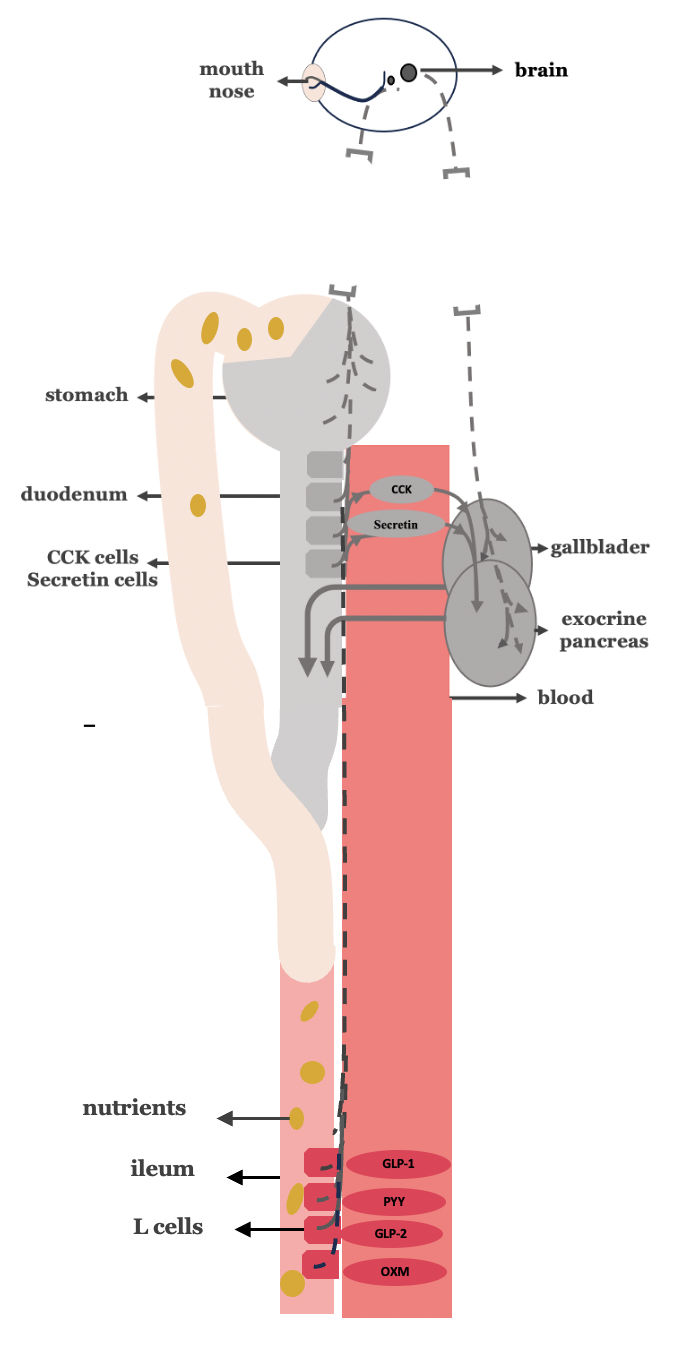
While gastric bypass alone inhibits biliopancreatic secretion by attenuating the gastric and intestinal phases, truncal vagotomy uniquely suppresses all three phases—cephalic, gastric, and intestinal—by eliminating vagal stimulation. Thus, the combination of truncal vagotomy with gastric bypass offers a synergistic approach, enhancing the overall inhibitory effect on biliopancreatic secretion and potentially leading to superior metabolic outcomes in type 2 diabetes mellitus compared to gastric bypass alone (Figure 4).
Given that longer disease duration and poor preoperative glycemic control are known to reduce remission rates and increase the risk of late relapse, we recommend considering bariatric-metabolic surgery—especially strategies involving truncal vagotomy—during the early stages of type 2 diabetes, when β-cell function remains partially reversible and insulin resistance is more amenable to intervention.
Figure 4. Truncal vagotomy in combination with gastric bypass.
-The grayish discoloration of the stomach reflects (a) the lack of food passage through the stomach as a result of gastric bypass, and (b) the consequent inhibition of the gastric phase of biliopancreatic secretion.
-The grayish discoloration of the proximal intestine (duodenum and proximal jejunum) reflects (a) the lack of nutrient passage through this region as a result of gastrointestinal bypass, and (b) the consequent inhibition of the intestinal phase of biliopancreatic secretion, primarily due to reduced stimulation of CCK- and secretin-secreting enteroendocrine cells.
-Truncal vagotomy, depicted in the figure as an interruption of the continuity of the vagus nerve, inhibits all three phases of biliopancreatic secretion (cephalic, gastric, and intestinal)
-The suppression of biliopancreatic secretion is illustrated using gray shading; accordingly, the gallbladder and the exocrine pancreas are also shown in gray to reflect their reduced functional activity.
-The red discoloration of the distal intestine(ileum) reflects the excessive stimulation of L-cells of the yndugested nuterients and the increased release of the GLP-1, GLP-2, PYY and oxymondulin.
-The red discoloration of the distal intestine (ileum) reflects the excessive stimulation of L-cells by the undigested nutrients, leading to increased release of GLP-1, GLP-2, PYY, and oxyntomodulin
-The mechanisms underlying these effects are illustrated in Figures 2 and 3
Potential drawbacks and limitations of adding truncal vagotomy in gastric bypass procedures.
Achieving complete truncal vagotomy is a technically demanding surgical procedure that requires meticulous exposure of the distal 5 cm of the esophagus, including the resection of extra-esophageal pre-aortic and hepatic branches(26). Due to the anatomical complexity and variability, incomplete nerve resection remains a significant challenge in the field and may compromise the intended metabolic benefits of truncal vagotomy. One of the most serious intraoperative risks associated with truncal vagotomy is esophageal perforation during dissection, which, although exceedingly rare, can lead to life-threatening complications (27). Additionally, the incorporation of truncal vagotomy may prolong operative time, particularly in patients with pre-existing perioperative morbidity, increasing the overall surgical risk.
From a postoperative perspective, truncal vagotomy carries its own set of potential complications. A notable concern is post-vagotomy diarrhea, which is clinically significant in approximately 5% to 10% of patients and may affect quality of life. Furthermore, when truncal vagotomy is combined with gastric bypass, it may exacerbate known complications of bypass procedures, such as steatorrhea, anemia, and osteoporosis, due to increased malabsorption. Interestingly, the incidence of dumping syndrome appears to be lower following truncal vagotomy (20%) compared to gastric bypass alone (40%), suggesting that vagal denervation may offer some protective effect against this syndrome (28).
Conclusions
Various types of bariatric-metabolic surgery in subjects with morbid obesity frequently lead to remission of co-existing diabetes mellitus, often even before significant body weight loss occurs. This effect may result from the correction of amplified digestion and the subsequent reduction in excessive nutrient absorption observed in patients with type 2 diabetes mellitus.
Since amplified digestion is driven by both enhanced vagal stimulation and hormonally mediated activation of biliopancreatic secretion via cholecystokinin and secretin, a dedicated antidiabetic surgical approach should include two key components: (a) truncal vagotomy to reverse heightened vagal parasympathetic activity, and (b) surgical bypass of the proximal intestine to prevent nutrient-induced overstimulation of hormone-secreting cells located predominantly in that segment.
We present a strong rationale for the integration of truncal vagotomy into existing gastrointestinal bypass procedures as a method to amplify the antidiabetic effects of bariatric-metabolic surgery. Truncal vagotomy suppresses vagally mediated digestive activity throughout the gastrointestinal tract, thereby significantly enhancing the inhibition of biliopancreatic secretion. In our proposed approach, truncal vagotomy is not limited by BMI as an inclusion or exclusion criterion; rather, BMI helps determine the most appropriate type of combined surgical intervention. Accordingly, we propose studying the addition of truncal vagotomy to gastric bypass in carefully selected patients with type 2 diabetes—specifically those with a short disease duration, satisfactory preoperative glycemic control, moderately elevated HbA1c levels, and reversible β-cell dysfunction—within the framework of well-designed randomized clinical trials. The trial VagusSx has been developed to explore the combined use of truncal vagotomy and gastric bypass as a unified strategy for achieving durable, long-term remission of type 2 diabetes.
Although truncal vagotomy introduces technical complexity and carries potential limitations and risks, such as extended operative time or postoperative complications, it remains a familiar procedure to upper gastrointestinal surgeons. Truncal vagotomy has historically been applied in the management of peptic ulcer disease and continues to be used in certain gastric cancer surgeries. We believe that, despite its invasiveness, truncal vagotomy offers a powerful tool for metabolic modulation in diabetes surgery. Looking forward, we anticipate that the goal of reducing biliopancreatic secretion as a therapeutic strategy for type 2 diabetes may eventually be achieved through less invasive, nonsurgical methods—though, at present, truncal vagotomy remains a viable and promising adjunct in surgical diabetes care.