Abstract
Several research studies have showed that the significant improvement or even remission of type 2 diabetes mellitus after bariatric-metabolic surgical procedures, especially those involving by-passing of food from the proximal intestine, is due to mechanisms beyond body weight loss and food restriction. Τwo models have been put forward to explain the weight-independent antidiabetes mechanisms of the gastrointestinal by-pass procedures. According to the ‘distal intestinal hypothesis’, the increased postprandial secretion of L-cell incretin-peptides, such as GLP-1, due to enhanced distal-intestinal nutrient delivery, increases insulin secretion, insulin action, and β-cell function. b) According to the anti-incretin theory, excess of ‘anti-incretin neuroendocrine signals’, primarily abundant in the proximal intestine and stimulated by macronutrient composition or chemical additives of modern diets, might cause insulin resistance, reduced insulin secretion, and β-cell depletion, leading to T2DM.
The reduction of postprandial secretion of excess incretins as a result of the exclusion of the passage of nutrients from the proximal intestine, improves the sensitivity to insulin, increases insulin secretion, and β-cell function. Based on the above model, we have suggested that the anti-incretin signals of the proximal intestine could be ‘specific digestive gastrointestinal neuro-endocrine signals’. Τhe addition of truncal vagotomy to the gastric by-pass surgery, which we proposed as a dedicated anti- diabetes surgery, aims to reduce the excess of the digestive neuroendocrine signals, encountered in type 2 diabetes.
The birth of diabetes surgery!
In 1995, Dr Pories published a landmark article with the provocative title: “Who would have thought of it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus” (1). At that time, Pories observed a drastic improvement in blood glucose levels after gastric bypass in obese subjects who had diabetes or impaired glucose tolerance. This was the earliest indication of a potential diabetes cure by surgury. Pories commented: ‘Non-insulin-dependent diabetes mellitus is no longer an uncontrollable disease. A return to normal levels of plasma glucose, insulin, and glycosylated hemoglobin are now attainable with gastric by-pass in the majority of morbidly obese diabetic patients, especially if the therapy can be initiated in the first 2 years after the diagnosis of the disease. Why the operation controls diabetes so well is not clear, but the major reason appears to be the reduction of caloric intake.’ Since then, a plethora of scientific studies -retrospective, observational, randomized controlled, meta-analytical- have followed, focusing on the remission of type 2 diabetes mellitus after bariatric-metabolic surgeries.
Established Bariatric-Metabolic Surgery procedures
The four most commonly used bariatric-metabolic surgical procedures are shown in the figure and summarized below:
Α. Adjustable gastric banding (AGB), developed in the mid-1990s, involves encircling the upper part of the stomach with an inflatable silicon ring, creating a smaller stomach pouch. The device is adjusted to optimize the diameter of a tight aperture that hinders food flow (2, 3) (Fig. A).
Β.Sleeve gastrectomy (SG) is the most commonly performed bariatric-metabolic surgery procedure worldwide since 2005. It is based on the removal of most of the stomach, leaving behind a small, tubular portion like a sleeve. This procedure reduces the functional capacity of the stomach, while it preserves the normal route of nutrients through the gastrointestinal tract (3, 4) (Fig. B)
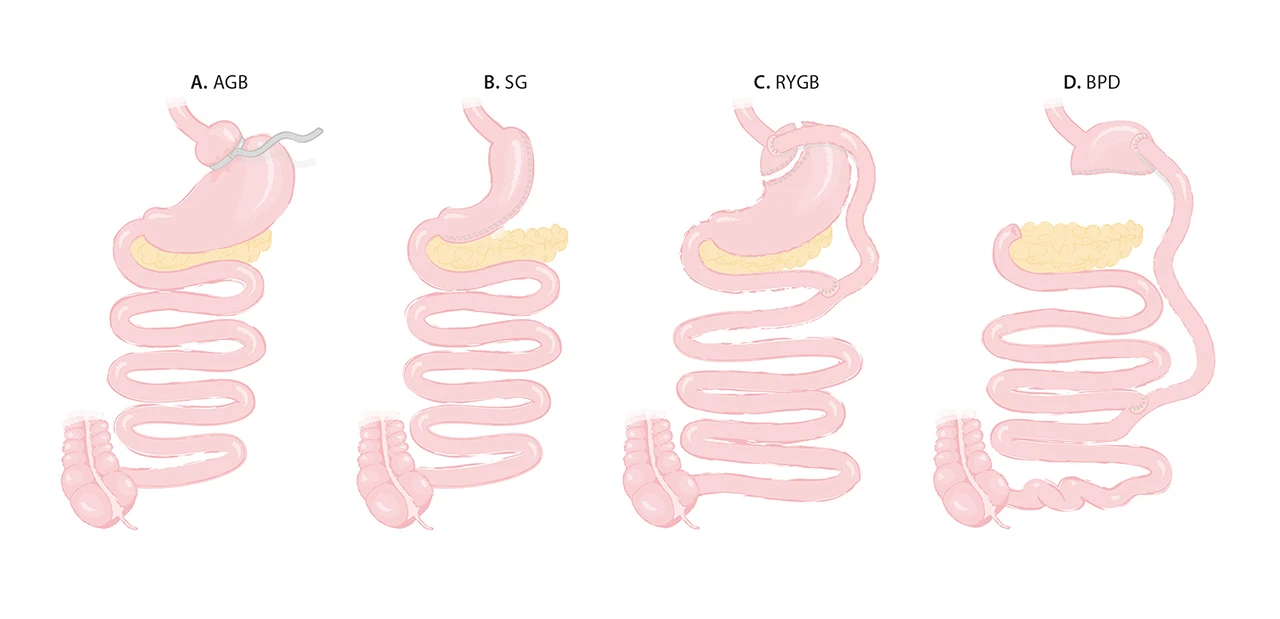
C. Roux-en-Y gastric bypass (RYGB), first described in 1967 as a mixed technique combining both gastric and small bowel mechanisms (3, 5). The stomach is divided into two compartments, leaving only the small upper part in digestive continuity. After gastric by-pass, ingested food bypasses ̴ 95 % of the stomach, the entire duodenum and a small portion of the jejunum (Fig. C).
D. Biliopancreatic diversion (BPD), developed in 1979, consists of a distal gastrectomy that leaves behind a functional upper stomach (3, 6). Food passes to the ileum, bypassing a large part of the small intestine (duodenum, jejunum, and a portion of the proximal ileum), “purposefully” causing some malabsorption (Fig. D).
Diabetes surgery: Better diabetes outcomes by gastrointestinal by-pass procedures.
Several studies have provided evidence that among bariatric-metabolic surgery procedures, those that involve intestinal by-pass, like gastric bypass and biliopancreatic diversion are the most effective on diabetes remission, with the lowest recurrence rates. The degree of decreasing efficacy for body weight loss and diabetes remission with low recurrence rates among the surgical procedures is: BPD > RYGB>SG > AGB. In our publication, in 2022, we provided a comparative table of short- and long-term outcomes of type 2 diabetes remission and relapse among the most commonly used bariatric-metabolic operations (7).
A study used the national database of the ‘Bariatric Surgery Center of Excellence Program’ of the American Society of Metabolic and Bariatric Surgery, found the following: Among 23.106 patients with metabolic syndrome who underwent bariatric surgery, the 12-month remission rate of type 2 diabetes was least for gastric banding (28%), followed by sleeve gastrectomy 52%. The procedures involning intestinal by-pass like RYGB and the BPD had the best rates of diabetes remission compared with the other procedures after one year of follow up (62% and 74%, respectively) (8). Similar results were also reported by other researchers in both retrospective (9) and prospective studies (10), after 2 and 3 years of follow-up, respectively.
In studies with 5 and 8 years of follow-ups, gastric bypass continued to be superior to sleeve gastrectomy not only in the rates of diabetes remission but also in the rates of its recurrence (11–13).
Diabetes surgery: evidence of body weight-independent anti-diabetic effects after gastrointestinal surgeries
Following the encouraging antidiabetic results of bariatric surgery, interest has rapidly mounted in the mechanisms by which the gastrointestinal by-pass procedures ameliorate type 2 diabetes, regardless of the body weight loss and diminished caloric intake. Indeed, growing evidence indicates that the remarkable anti-diabetic effects is the result not only of weight loss but also of the so-called “weight-independent anti-diabetic mechanisms”. The evidence in favour of the existence of these mechanisms comes from the following observations:
I.Early postoperative remission of type 2 diabetes mellitus.
Many studies have demostrated that the resolution of diabetes occurs within days to weeks following bariatric-metabolic surgery, long before body weight loss has occurred. Pories et al, observed that, among 330 obese patients with type 2 diabetes, diabetes was resolved within days after gastric bypass surgery! In a study including 240 obese patients with type 2 diabetes who underwent gastric by-pass, diabetic medications (oral agents or insulin) were discontinued immediately after hospital discharge and before significant body weight loss occurred in 30% of the patients (14). In a prospective study by Rubino et al, the patients with type 2 diabetes mellitus were euglycemic without medications within 3 weeks of gastric by-pass surgery, although the mean body mass index (BMI) was not yet significantly changed (15). This remarkably early postoperative diabetes remission reveals the role of a specific anti-diabetic mechanism (s) beyong body weight-loss and food restriction.
II. Greater diabetes resolution with gastric by-pass induced body weight loss than with equivalent body weight loss from other interventions.
Lee et al. found that among 60 obese patients with type 2 diabetes randomly assigned to undergo gastric by-pass or sleeve gastrectomy, diabetes remission was far greater among the gastric by-pass patients, despite a similar body weight loss in both groups at six months (16). Pattou et al. compared obese patients with type 2 diabetes with an equivalent 10% body weight loss, following gastric banding vs. gastric by-pass. They demonstrated that diabetes remission rates were significantly greater after gastric by-pass than after gastric banding (49% vs. 25%, respectively). The difference was attributed to the dramatic restoration of β-cell function, closely linked with the impressive increase in GLP-1 levels after gastric by-pass (17, 18).
Laferrere et al (19) showed that, after a similar bodyweight loss of 10 kg, the gastric by-pass group of obese patients with type 2 diabetes mellitus had a much greater improvement in glucose tolerance than the matched control group that followed a low-calorie diet. Furthermore, this study demonstrated that the anti-diabetic effects were the result of a six-fold increase in GLP-1 levels in the surgical group, in contrast to no increase in the diet group.
III. Diabetes remission after proximal intestinal by-pass in lean subjects
The direct anti-diabetic effect of the gastrointestinal by-pass procedure on type 2 diabetes mellitus was initially suggested by Rubino et al. based on an experiment conducted on lean diabetic rats (20). From our point of view, this experiment represents a milestone in the history of diabetes surgery and a source of inspiration for the development of our own theory (7). The experimental animals underwent duodenal-jejunal bypass, which comprises a bypass of the proximal intestine similar to that in a standard gastric by-pass, without gastric restriction. These rats experienced substantially greater improvement in glucose homeostasis than matched control rats who underwent sham surgery, diet restriction or therapy with insulin-sensitising drugs. Subsequent experiments by the same research group revealed that bypassing a short segment of proximal intestine directly ameliorates type 2 diabetes, independently of its effects on food intake or body weight (21). These findings have also been substantially replicated by experiments using duodenal-jejunal by-pass in humans with BMI as low as 24kg/m2 (22, 23). However, a Chinese study showed that duodenal-jejunal by-pass can improve glycemic control in lean (BMI: 24kg/m2) patients with type 2 diabetes but it cannot lead to complete remission of the disease (24).
IV. Diabetes remission after gastrectomies in non-obese patients.
Consistent observations of type 2 diabetes remission have also been reported as unexpected outcomes after gastrectomies in patients with concurrent gastric cancer and type 2 diabetes. These surgical procedures involve partial or total resection of the stomach, often combined with a proximal intestinal by-pass analogous to that in gastric by-pass. The literature of this field is impressive! The surgery of gastric cancer is not only a radical tumor resection surgery but also a surgery for diabetes remission! Note that these patients who undergo surgery not for morbid obesity but for stomach malignancies, are mostly lean.
Zhu et al. found that 292 patients with concurrent gastric cancer and type 2 diabetes, who underwent curative gastric resection, achieved complete remission of diabetes at a rate of 30 to 90%, depending on the type of gastrectomy and the reconstruction method. The patients who underwent gastrectomy had higher rates of diabetes remission when proximal intestinal bypass was involved than without by-pass (77% vs. 29%, respectively). The patients who underwent total gastrectomy with intestinal by-pass exhibited the highest remission rates, reaching 90%. What is impressive is that the BMI of these patients was as low as 22kg/m2, with no significant reduction up to 2 years postoperatively (25).
Similarly, a retrospective study by Lee et al., showed that lean patients with gastric cancer and type 2 diabetes who received gastrectomy showed remission or improvenent of diabetes up to 75% at a 5-year follow-up. Diabetes remission rates were higher in the group with intestinal by-pass compared than the group without it (67.2% vs. 49.5%, respectively). Τotal gastrectomy plus intestinal by-pass had the higher remission rate of 50% (26).
A systematic review and meta-analysis of a total of nine studies, including 1424 patients, is in agreement with the results of the above studies: A. The total gastrectomy group had better diabetes remission than the partial gastrectomy group. B. The group with a proximal intestinal by-pass as the reconstruction method, had better diabetes remission rates than the non-by-pass group. C. Total gastrectomy with proximal intestinal by-pass had the best diabetes remission rate after at least 1 year of follow- up after gastrectomy (27).
What hypotheses explain the direct effect of diabetes remission in diabetes surgery?
Two hypotheses have been proposed to explain the early effects of gastrointestinal procedures- especially those including proximal intestinal by-pass- on type 2 diabetes remission (28). According to the ‘lower intestinal hypothesis (also known as ‘distal’ or ‘hindgut’ hypothesis), the rapid delivery of the nutrients to the lower intestine increases the stimulation of L-cells (29). The L-cells are present at high density in the distal gut and secrete the incretins in the intestinal lumen, in response to nutrients.
The incretins, with GLP-1 as their main representative, are hormones that stimulate insulin secretion and/or action. The increased stimulation of L-cell and the subsequent increase in incretin secretion, ultimately lead to enhancement of insulin release and/or insulin action. Τhe significant postoperative increase and maintenance of GLP-1 levels has been confirmed by many clinical studies (30). However, one asks, do elevated levels of GLP-1 constitute the body weight-independed mechanism that triggers the postoperative remission of diabetes? If this is proven true, the hindgut hypothesis would spur further research on methods to enhance signaling by GLP-1 (or other distal gut hormones) to treat type 2 diabetes.
On the other hand, the ‘upper intestinal hypothesis, also defined as ‘proximal’ of ‘foregut’ hypothesis, postulates that the exclusion of the proximal intestine from the transit of nutrients, possibly prevents the secretion of putative signals that promote insulin resistance, reduce insulin secretion and lead to type 2 diabetes mellitus. Reduction of the amount of these signals would increase insulin secretion and/or action, thereby improving symptoms of type 2 diabetes. Although no obvious candidate molecules can be identified with current knowledge, if proven true, this hypothesis might open new avenues in the search for the cause and cures of diabetes (21, 31).
The anti-incretin theory behind the discovery of diabetes surgery.
The anti-incretin theory presupposes the axiom of the ‘proximal hypothesis’. This theory, developed by Rubino in 2002 (32) and progressively refined (31), could explain both the effects of gastrointestinal surgery on type 2 diabetes mellitus and the role of the gastrointestinal tract in the physiology and pathophysiology of glucose homeostasis.
The ‘anti-incretin’ theory suggests the existence of nutritionally-stimulated gastrointestinal neuroendocrine signals that antagonize the effects of incretins. A normal, physiologic balance between incretins and ‘anti- incretins’ would ensure normal excursions of blood glucose and proper β-cell function. An excess of anti-incretin signals, possibly stimulated by the specific macronutrient composition of modern diet or chemical additives, could disrupt the incretin-anti-incretin homeostatic mechanism, causing insulin resistance, reduced insulin secretion, and β-cell depletion, leading to T2D. Reduction of nutrient stimuli on the gut by a drastic decrease in food intake (i. e., very low calorie diet), expedited gastrointestinal transit (i. e., such as after sleeve gastrectomy), or, more radically, through the exclusion of large portions of the upper small bowel from nutrient transit (i. e., gastric by-pass) could reduce excess anti-incretin and restore appropriate incretins/anti-incretins balance, explaining, thus, the clinical improvement in type 2 diabetes.
Diabetes Surgery: Is the gut the ‘key’ for the treatment of diabetes?
In 2014, Rubino published an article, entitled ‘Is the Gut the “Sweet Spot” for the Treatment of Diabetes?’ In this article, he proposed an insightful reasoning. Since the gastrointestinal procedures can lead to type 2 diabetes remission, then the gut could be the key point for treatment of the disease.
This proposal was based on his revolutionary experiment in rats (20). The duodenal-jejunal intestinal bypass in lean diabetic rats resulted in improved glucose tolerance compared to all control groups, while the restoration of duodenal passage, in the same rats, reestablished impaired glucose tolerance! These surprising findings indicate that the proximal intestine triggers -through potentially undiscovered factors- the pathogenesis of type 2 diabetes, while the proximal intestinal by-pass improves the disease.
Τhe anti-incretin theory as a source of inspiration for our own theory on diabetes surgery.
Based on the anti-incretin theory, we considered that the balance between incretins and ‘anti-incretins’ could be described as an “equation” in which the anti-incretin factors are the “unknown variables” (33). After a thorough research study of the physiology and pathophysiology of the gastrointestinal truct, we concluded that the neuroendocrine signals responsible for food digestion – primary triggered by the passage of food through the proximal intestine- could be considered “anti-incretins”. In our recent publication (7), we described in detail the inductive reasoning that led us to the above conclusion.
Digestion is largery carried out by the nutrient-stimulated neuroendocrine cells, primarily represented by the CCK cells that are most abundant in duodemun and proximal jejunum. These cells send endocrine and neural signals to the pancreas and gallbladder in order to secrete pancreatic enzymes and bile, respectively. These, in turn, break down carbohydrates, lipids and proteins, converting them respectively into the absorbable products: glucose, free fatty acids and amino acids.
Building upon the anti-incretin theory, we consider that glucose homeostasis is achieved through the balance between the digestive neuroendocrine signals that ultimately lead to the entry of glucose into the blood and the incretins responsible for the insulin-dependent blood glucose disposal. Cells are over-stimulated by high glycemic index and high fat diet, due to the adaptation of the intestine to increased digestion. The over-stimulated cells send excessive digestive neuroendocrine signals to the pancreas and the gallbladder. The disruption of the balance between incretin-mediated digestive neurohormonal signals leads to amplified digestion and consequently to increased nutrient absorption.
The chronic exposure of tissues to high plasma levels of glucose, fatty acids and amino acids leading to tissue resistance to the actions of insulin and, at a later stage, β-cell dysfunction, reduction of insulin release and type 2 diabetes. Conversely, the exclusion of the proximal small bowel from nutrient transit could reduce the excess of the digestive signals by the neuroendocrine cells restoring the balance with the incretins. As a result, the amplified digestion and nutrient absorption return to normal levels, reversing the insulin resistance and the reduced insulin secretion, improving thus type 2 diabetes.
Conclusions and comments
Given the advancements in diabetes care, metabolic surgery can now be offered as a treatment option to selected patients with type 2 diabetes mellitus (34). In the last 20 years, extensive research is being conducted to elucidate the body weight-independent mechanisms underlying the beneficial effects of gastrointestinal surgery on type 2 diabetes. The clarification of these mechanisms may facilitate the design of dedicated antidiabetes gastrointestinal interventions and the identification of targets for novel pharmaceuticals that might replicate some of the dramatic effects of the surgical operations. It will also open new horizons in the understanding of the pathophysiology of the disease (35).
Specifically, the anti-incretin theory provides a rational and intergrated theoretical model to explain the physiology and pathophysiology of glucose metabolism, as well as the effects of the various interventions, including gastrointestinal surgery and diet. Based on this model, we suggested ‘the digestive gastrointestinal neuro-endocrine signals’ as anti-incretins (7). Τhe addition of truncal vagotomy to the gastric bypass, which we proposed as a dedicated antidiabetes gastrointestinal manipulation, aims to reduce the excess of the digestive neuroendocrine signals encountered in type 2 diabetes.